0. A Review on the Expanded Applications of mRNA Vaccine Platforms in Preventing and Controlling Emerging Infectious Diseases
1. Introduction
This review provides a comprehensive examination of mRNA vaccine platforms, focusing on their expanded utility in preventing and controlling emerging infectious diseases. Traditional vaccine development methods often face significant limitations, primarily their slow response times, which prove inadequate for rapidly evolving outbreaks .
The foundational principles of mRNA technology offer a transformative shift by enabling rapid design, scalability, and adaptability, positioning it as a pivotal solution for global health security .
The core mechanism of mRNA vaccines involves delivering synthetic mRNA sequences, encapsulated within lipid nanoparticles (LNPs), into host cells . These mRNA sequences then instruct the cellular machinery to produce specific viral antigens, which subsequently elicit a robust, targeted immune response, preparing the host to combat future infections . This approach contrasts sharply with traditional vaccine platforms, which often involve complex and time-consuming manufacturing processes, handling infectious agents, or requiring strong adjuvants to induce sufficient immunity .
The transformative potential of mRNA vaccines lies in their unprecedented speed and flexibility. Once a pathogen's genetic sequence is known, the corresponding mRNA sequence can be rapidly synthesized, drastically shortening the development timeline from years to weeks . This rapid design capability, coupled with highly scalable in vitro manufacturing, makes mRNA technology uniquely suited for swift responses to emerging threats and global pandemic preparedness . Furthermore, mRNA vaccines elicit strong, durable humoral and cellular immune responses while acting as intrinsic adjuvants, contributing to their high efficacy .
The successful deployment of mRNA vaccines during the COVID-19 pandemic served as a critical validation of this platform's capabilities, demonstrating its efficacy, safety profile, and rapid response potential . This review will detail the evolution of mRNA vaccine technology, including advancements in design and delivery mechanisms, and explore its significant role in global health security against emerging infectious diseases, while also acknowledging broader applications such as in cancer immunotherapy . Subsequent sections will delve into specific applications, challenges, and future opportunities for this innovative platform.
1.1 Background and Significance of mRNA Vaccine Technology
Messenger RNA (mRNA) vaccine technology represents a transformative advancement in vaccinology, fundamentally altering the paradigm for developing and deploying immunizations, especially in response to emerging infectious diseases . The core principle of this technology involves delivering synthetic mRNA sequences, which encode specific viral antigens, into host cells . Upon entry into the host cell, the cellular machinery, specifically ribosomes, translates these mRNA instructions into proteins that mimic the pathogen's antigens. These newly synthesized antigens then trigger a robust and targeted immune response within the host, preparing the body to recognize and combat future encounters with the actual pathogen .
A critical component enabling the success and safety of mRNA vaccines is the encapsulation of the mRNA molecule within lipid nanoparticles (LNPs) . These LNPs serve dual purposes: they protect the fragile mRNA from degradation by nucleases and facilitate its efficient delivery and uptake into host cells . Furthermore, the mRNA molecule itself is engineered with specific structural elements, including a 5′ cap, a poly(A) tail, and untranslated regions, to enhance its stability, optimize translation efficiency, and modulate its immunogenicity . Early challenges related to mRNA instability and the triggering of unwanted innate immune responses were meticulously addressed through decades of research, leading to the development of stable, less immunogenic mRNA molecules suitable for clinical application . The most prevalent form of mRNA vaccine currently utilizes linear, non-replicating mRNA, designed for administration via intramuscular, subcutaneous, or intradermal injection .
The attributes that position mRNA vaccine technology as particularly suitable for rapid response to emerging infectious diseases are multifaceted. Firstly, its rapid design capability is unparalleled . Once the genetic sequence of a new pathogen is identified, the corresponding mRNA sequence encoding the target antigen can be rapidly synthesized in vitro, significantly shortening the development timeline from months or years to weeks. This inherent flexibility in antigen design allows for broad applicability against a wide spectrum of pathogens, including viruses, bacteria, and parasites .
Secondly, mRNA vaccine manufacturing is highly scalable . The in vitro synthesis process, which does not require cell culture or complex purification steps associated with traditional vaccine production, enables rapid and large-scale manufacturing of vaccine doses to meet global demand during a pandemic . This contrasts sharply with the often-lengthy and resource-intensive production processes of conventional vaccines.
Thirdly, mRNA vaccines exhibit strong immunogenicity, eliciting robust and durable immune responses, including both humoral (antibody-mediated) and cellular (T-cell mediated) immunity, while also generating immunological memory . The mRNA molecule itself acts as an intrinsic adjuvant due to its immunostimulatory properties, activating innate immune cells like dendritic cells and macrophages at the injection site. These activated cells then migrate to lymphoid organs, priming and activating T and B cells to orchestrate a comprehensive adaptive immune response . This dual action as both immunogen and adjuvant contributes to the high efficacy observed with these vaccines .
Comparing mRNA vaccine technology with conventional vaccine approaches highlights its distinct advantages and underscores its significance in the contemporary landscape of vaccine development. Traditional vaccine platforms, such as inactivated pathogens, live-attenuated vaccines, subunit vaccines, recombinant viral vectors, and DNA-based vaccines, often involve more complex and time-consuming manufacturing processes . For instance, inactivated and live-attenuated vaccines require handling infectious agents, posing safety concerns during production and potentially for the vaccine recipient if attenuation is incomplete . Subunit vaccines, while safer, often necessitate extensive purification steps and may require strong adjuvants to elicit adequate immune responses. Recombinant viral vector vaccines face challenges related to pre-existing immunity to the vector itself, which can reduce vaccine efficacy. DNA vaccines, while sharing some advantages with mRNA in terms of genetic material delivery, carry a theoretical risk of genomic integration, a concern that mRNA vaccines circumvent as they do not integrate into the host genome .
The successful development and deployment of mRNA vaccines against SARS-CoV-2 during the COVID-19 pandemic served as a pivotal validation of this platform's potential for rapid response, high efficacy, and an acceptable safety profile . This unprecedented success has not only driven increased interest but also demonstrated the platform's ability to offer a reactive and proactive approach to disease prevention, positioning mRNA technology at the forefront of modern vaccinology for tackling emerging infectious diseases .
1.2 Scope and Objectives of the Review
This review comprehensively examines the expanded applications of mRNA vaccine platforms, specifically focusing on their utility in preventing and controlling emerging infectious diseases (EIDs). The scope encompasses the foundational elements of mRNA technology, its inherent advantages over conventional vaccine platforms, and the significant advancements in design, delivery mechanisms, and clinical trials . While acknowledging the broader applications of mRNA technology, such as in cancer immunotherapy and autoimmune disorders, the primary emphasis of this review remains on infectious diseases, particularly in the context of pandemic preparedness and response . This allows for an in-depth analysis of their unique benefits and the challenges public health officials face in their widespread implementation.
The review aims to answer several critical questions, serving as a roadmap for the subsequent sections. Firstly, it seeks to elucidate the current advancements in mRNA vaccines for preventing EIDs, summarizing the progress made and identifying key successes in this field . This involves exploring the types of mRNA vaccines under development, their sophisticated delivery systems, and the robust immune responses they elicit . Specifically, the review will highlight how recent innovations in mRNA vaccine design, such as modifications to increase stability and translation efficiency, have contributed to their efficacy against various pathogens . For instance, the use of nucleoside-modified mRNA and lipid nanoparticle (LNP) delivery systems has been instrumental in enhancing both the safety and immunogenicity profiles of these vaccines, a significant leap from traditional vaccine platforms .
Secondly, the review will meticulously examine the limitations and future prospects of this technology . This includes addressing critical challenges such as the stability of mRNA molecules, the optimization of immune responses, and ensuring global accessibility and equitable distribution . The review will delve into strategies for overcoming these hurdles, such as developing novel formulations for improved thermostability, which would significantly reduce cold chain requirements and facilitate deployment in resource-limited settings . Furthermore, it will explore the potential for developing next-generation mRNA vaccines with enhanced efficacy and broader protective capabilities, including those targeting multiple pathogens or rapidly mutating viruses .
The scope also extends to evaluating the role of mRNA vaccines in future pandemic preparedness. The rapid development and deployment of mRNA vaccines during the COVID-19 pandemic underscored their potential for accelerating vaccine development cycles in response to novel threats . This section will discuss how the modularity and rapid manufacturing capabilities of mRNA platforms position them as a cornerstone for future pandemic responses, enabling quicker antigen updates and scale-up compared to traditional methods . For example, the ability to rapidly synthesize mRNA sequences corresponding to new viral variants allows for agile vaccine adjustments, a critical feature for mitigating the impact of fast-evolving pathogens.
While the primary focus is on infectious diseases, it is pertinent to acknowledge that mRNA technology’s versatility extends to other significant therapeutic areas, such as oncology. Several sources highlight the dual application of mRNA platforms in both infectious disease prevention and cancer immunotherapy . This intersection underscores the broad potential of the technology, although specific detailed discussions on cancer immunotherapy will be beyond the core scope of this review. The objective is not to provide an exhaustive survey of all mRNA applications, but rather to concentrate on its expanded role in preventing and controlling emerging infectious diseases, offering a comprehensive analysis of the progress, challenges, and future trajectories within this specific domain.
This review will serve as a foundational resource for researchers, policymakers, and public health professionals, offering a structured understanding of the current state and future directions of mRNA vaccine technology in infectious disease control. The subsequent sections will systematically address the aforementioned objectives, starting with an overview of mRNA vaccine mechanisms, followed by detailed discussions on specific applications against various EIDs, and concluding with a synthesis of future opportunities and the remaining hurdles for widespread global adoption.
2. Evolution and Core Principles of mRNA Vaccine Platforms
The rapid ascent of messenger RNA (mRNA) vaccine technology, culminating in its pivotal role during the COVID-19 pandemic, is a testament to decades of foundational research and critical scientific breakthroughs . This section delves into the chronological development of mRNA vaccine platforms, elucidating key milestones that transformed a promising concept into a clinically viable immunization strategy. It further explores the molecular mechanisms and fundamental design principles underpinning mRNA vaccines, including the critical modifications and structural elements that dictate their stability, expression, and immunogenicity. Finally, a comparative analysis of different mRNA vaccine platforms, such as non-amplifying and self-amplifying mRNA, will highlight their respective advantages and disadvantages concerning antigen production, dose requirements, and manufacturing scalability, thereby providing a comprehensive understanding of the technology's adaptability and future potential in combating diverse emerging infectious diseases .
The historical development of mRNA vaccine technology commenced with early conceptualizations of mRNA as a therapeutic agent in the 1940s and 1990s, but faced considerable challenges related to inherent instability, susceptibility to degradation, inefficient delivery, and undesirable innate immune activation . Breakthroughs in the mid-2000s, particularly the incorporation of modified nucleosides like pseudouridine and the advent of lipid nanoparticle (LNP) delivery systems, proved transformative. These innovations significantly enhanced mRNA stability, reduced immunogenicity, and facilitated efficient cellular uptake, paving the way for clinical translation . The rapid development and deployment of mRNA vaccines during the COVID-19 pandemic served as a pivotal validation, showcasing the platform's unprecedented speed and efficacy in global health crises .
The molecular mechanisms of mRNA vaccines are based on delivering synthetic mRNA that encodes a target antigen, which is subsequently translated by host cell ribosomes to produce the antigen, thereby eliciting robust humoral and cellular immune responses . Key design principles involve optimizing mRNA sequence elements such as the 5′ cap, untranslated regions (UTRs), and poly-A tails to enhance translation efficiency and stability, alongside the crucial nucleoside modifications that reduce innate immune sensing .
Current mRNA vaccine platforms primarily comprise non-amplifying mRNA (NRM) and self-amplifying mRNA (SAM) . NRM vaccines encode only the target antigen, leading to a single round of protein production, while SAM vaccines incorporate a viral replicase gene, enabling intracellular amplification and sustained, higher antigen expression . LNPs are the cornerstone of mRNA vaccine delivery, encapsulating mRNA to protect it from degradation and facilitate cellular uptake and endosomal escape . LNPs are composed of ionizable lipids, cholesterol, phospholipids, and PEG-lipid conjugates, each contributing to stability, cellular entry, and immune modulation . Understanding these core principles and their evolution is crucial for appreciating the remarkable versatility and future potential of mRNA vaccine platforms in global health.
2.1 Historical Development and Milestones
The trajectory of messenger RNA (mRNA) vaccine research spans several decades, transitioning from foundational molecular biology discoveries to its current status as a rapidly deployable prophylactic and therapeutic platform. The concept of mRNA itself emerged in the early 1940s, laying the groundwork for subsequent therapeutic applications . Early in vivo applications of mRNA as a therapeutic agent were explored, such as the development of an mRNA vaccine for diabetes insipidus in rats via intrahypothalamic injection, highlighting initial considerations for direct therapeutic use .
Despite this early conceptualization, the practical application of mRNA in vaccines faced significant hurdles throughout the 1990s and subsequent decades . The primary limitations centered on mRNA's intrinsic instability, its susceptibility to degradation, challenges in efficient delivery to target cells, and the elicitation of potent innate immune responses that could degrade the mRNA or cause undesirable inflammatory reactions . These challenges rendered early mRNA vaccine approaches largely impractical for widespread clinical use .
A series of critical breakthroughs in the mid-2000s fundamentally transformed the viability of mRNA vaccine platforms. One pivotal innovation involved the chemical modification of nucleosides within the mRNA sequence. Specifically, the incorporation of modified nucleosides, such as pseudouridine, was instrumental in addressing two major limitations: enhancing mRNA stability and reducing its inherent immunogenicity . This modification allowed mRNA to bypass the body's innate immune sensors, preventing premature degradation and enabling more sustained and robust protein expression, which is crucial for effective antigen presentation and immune response generation .
Concurrent with nucleoside modifications, significant advancements in delivery systems proved equally critical. The development of lipid nanoparticles (LNPs) emerged as a transformative solution, addressing the persistent issues of inefficient mRNA transfer and protecting the delicate mRNA molecule from degradation in vivo . LNPs encapsulate the mRNA, facilitating its uptake by cells and enabling efficient translation into the desired antigenic protein . This encapsulation not only protects the mRNA but also aids in directing it to specific cell types, thereby optimizing the immune response . These improvements in LNP technology were fundamental in overcoming challenges related to both mRNA instability and low immunogenicity, making mRNA vaccines a powerful immunotherapeutic tool .
Beyond non-amplifying mRNA, early research also explored self-amplifying mRNA (saRNA) vaccines. For instance, a saRNA vaccine targeting H7N9 influenza was developed within eight days, demonstrating the platform's rapid response potential, though it did not proceed to clinical trials due to the absence of Good Manufacturing Practice (GMP) procedures at the time . This early work underscored the inherent speed advantage of mRNA technology in vaccine development.
The culmination of these decades of related research in molecular biology, lipid chemistry, microbiology, and immunology, alongside specific advancements in stabilizing viral spike proteins, led to the most significant milestone in mRNA vaccine history: the COVID-19 pandemic . The pandemic served as a catalyst for the rapid development and widespread global deployment of mRNA vaccines, marking a revolutionary breakthrough in vaccine science . The rapid approval and deployment of vaccines like Pfizer-BioNTech's BNT162b2 and Moderna's mRNA-1273 highlighted the critical role of mRNA technology in pandemic control and preparedness . This rapid success validated the decades of foundational research and established mRNA vaccine platforms as a cornerstone of modern immunization strategies, demonstrating their critical role in public health and pandemic response . The COVID-19 era solidified the platform's viability and ushered in a new era of immunization, positioning mRNA technology as a versatile and powerful tool for future vaccine development against emerging infectious diseases .
2.2 Molecular Mechanisms and Design Principles
mRNA vaccines operate on a fundamental principle: delivering synthetic mRNA strands encoding specific target antigens into host cells, which then leverage the cellular machinery to produce these proteins . Upon administration, these mRNA molecules are translated by host ribosomes into the intended viral proteins, such as the SARS-CoV-2 spike protein . This process mimics natural infection without recourse to live or inactivated viral components . The resulting antigens are subsequently presented to the immune system, thereby triggering robust humoral (antibody production) and cellular (cytotoxic T lymphocyte) immune responses, ultimately aiming to induce durable immunological memory . Specifically, this stimulates immune responses via MHC class I and II pathways, activating both CD8+ and CD4+ T cells . Following administration, mRNA vaccines enter the lymphatic system, reaching lymph nodes where they can transfect antigen-presenting cells (APCs), leading to the priming and activation of T and B cells .
Critical design considerations are paramount to influencing the stability, expression efficiency, and overall efficacy of mRNA vaccine platforms. These include optimizing the mRNA sequence, particularly through judicious codon usage, and modifying the untranslated regions (UTRs) and poly-A tails . The 5′ cap, for instance, is crucial for efficient translation and protection from degradation, while the poly(A) tail contributes to mRNA stability and translational efficiency . Codon optimization specifically aims to enhance translation efficiency by substituting codons with those more frequently used by human ribosomes, thereby increasing the rate of protein synthesis .
Furthermore, nucleoside modifications, such as the incorporation of N(1)-methylpseudouridine (), are employed to significantly improve protein expression and concurrently reduce the intrinsic immunogenicity of the mRNA molecule itself . This reduction in immunogenicity is crucial as unmodified mRNA can trigger innate immune sensors, leading to inflammatory reactions that could diminish antigen expression and vaccine efficacy . The mRNA molecule itself, particularly its structure and modifications, can also function as an adjuvant, further augmenting the immune response .
Two principal mRNA vaccine platforms exist: non-amplifying mRNA (nrRNA) and self-amplifying mRNA (saRNA). Non-replicating mRNA, exemplified by the approved COVID-19 vaccines, directly encodes the target antigen and relies on the host cell's translational machinery for a single round of protein production . In contrast, self-amplifying mRNA vaccines are derived from alphavirus genomes and incorporate a viral replicase gene. This unique feature allows the mRNA to amplify intracellularly, leading to significantly higher and sustained antigen expression compared to non-amplifying platforms . The enhanced antigen production from saRNA platforms is hypothesized to induce more potent and potentially longer-lasting immune responses, offering an advantage, particularly for targets requiring strong or sustained immunity. Beyond these, emerging categories include trans-amplifying mRNA, delivered as two separate mRNA molecules, and circular mRNA, which offers superior stability .
These design choices profoundly impact immunogenicity and efficacy across various viral targets. For instance, the use of nucleoside modifications, while reducing inflammatory reactions and innate immune sensing, must be carefully balanced to ensure sufficient innate immune activation for an effective adaptive immune response . High antigen expression, facilitated by self-amplifying mRNA or optimized non-amplifying mRNA, generally correlates with stronger immune responses. However, excessive or prolonged antigen expression could also potentially lead to immune tolerance or exhaustion in specific contexts, though current research largely points towards enhanced immunogenicity. The choice between non-amplifying and self-amplifying platforms often depends on the specific immunological requirements of the target pathogen. For rapid response to emerging threats like SARS-CoV-2, where widespread and robust immunity is needed quickly, non-amplifying mRNA proved highly effective due to its rapid development and deployment . For other pathogens requiring sustained high-level immunity or where lower doses are desirable to reduce reactogenicity, self-amplifying mRNA platforms may offer advantages due to their ability to produce more antigen from less initial mRNA . The systematic optimization of mRNA sequence elements, including the 5' cap, poly(A) tail, UTRs, and coding sequences, directly underpins the technological success of mRNA vaccine design for infectious diseases, enabling precise control over antigen expression and immune activation while mitigating unwanted immunological challenges.
2.3 Delivery Systems and Formulation Strategies
The instability and negative charge of messenger RNA (mRNA) molecules are inherent challenges that necessitate sophisticated delivery systems to facilitate their entry into target cells and protect them from degradation by nucleases . The success of mRNA vaccine platforms critically hinges on the efficiency and safety of these delivery mechanisms .
Historically, several techniques have been employed for nucleic acid delivery, including physical methods such as electroporation and gene guns, as well as biological approaches like ex vivo transfection . While these methods can achieve delivery, they often come with significant limitations. Electroporation, for instance, requires specialized equipment and can induce cell damage, while ex vivo transfection is labor-intensive and primarily suitable for applications where cells are treated outside the body before reintroduction . These constraints render them less ideal for widespread in vivo vaccine administration, particularly for large-scale public health interventions .
In contrast, lipid nanoparticles (LNPs) have emerged as the predominant and most clinically advanced in vivo delivery system for mRNA vaccines . The success of LNP technology is attributed to its multifaceted protective and facilitating roles. LNPs encapsulate the synthetic mRNA strands, shielding them from enzymatic degradation by nucleases present in biological fluids, thereby preserving their integrity until they reach the target cells . Furthermore, their lipidic composition enables efficient cellular uptake, primarily through endocytosis, after which they facilitate the crucial step of endosomal escape, releasing the mRNA into the cytoplasm where it can be translated into the target protein . This endosomal escape is vital, as it bypasses the lysosomal degradation pathway that would otherwise diminish mRNA efficacy.
The typical composition of LNPs includes four key components: ionizable lipids, cholesterol, phospholipids, and polyethylene glycol (PEG)-lipid conjugates . Ionizable lipids are critical because their charge can be manipulated based on pH, allowing them to be positively charged for mRNA encapsulation in acidic environments and then becoming neutral at physiological pH, which aids in endosomal escape and reduces systemic toxicity. Cholesterol contributes to the structural integrity and stability of the LNP membrane, while phospholipids aid in the formation of the lipid bilayer. PEG-lipid conjugates provide a steric barrier, preventing LNP aggregation and prolonging their circulation time in the bloodstream, which is essential for systemic delivery and improved biodistribution .
Beyond their role in mRNA protection and cellular delivery, LNPs also play a significant role in modulating the immune response. By encapsulating mRNA, LNPs prevent its premature recognition by endosomal pattern recognition receptors (PRRs) like Toll-like receptor 3 (TLR3) and TLR7, which could otherwise lead to an undesirable overactivation of the innate immune system . This shielding effect helps to balance the immune activation, ensuring a more controlled and effective adaptive immune response. While LNPs prevent overactivation by certain PRRs, it is also noted that modifications to mRNA, such as nucleoside incorporation or the addition of dsRNA regions, can enhance immune activation through interaction with other PRRs like RIG-I, leading to increased cytokine production and dendritic cell activation, thereby contributing to the overall vaccine efficacy .
Despite the clear advantages of LNPs, research continues into alternative and complementary delivery systems to further enhance efficacy, reduce potential side effects, and expand the applicability of mRNA vaccines. These include polymeric nanoparticles, such as those made from polyethyleneimine (PEI), dendrimers, and cationic nanoemulsions . Each of these alternative systems presents its own unique set of advantages and disadvantages. For instance, polymers like PEI offer versatility in terms of structural modification and can form stable complexes with mRNA, but their potential for cytotoxicity can be a concern. Dendrimers, highly branched macromolecules, provide precise control over their size and surface chemistry, potentially leading to improved targeting and cellular uptake, but their synthesis can be complex and costly. Cationic nanoemulsions offer good stability and encapsulation efficiency. The ongoing exploration of these diverse delivery vehicles aims to address specific challenges, such as targeting particular cell types, improving stability in various physiological conditions, and mitigating potential adverse reactions, thereby further broadening the therapeutic window for mRNA-based interventions . The optimization of these formulation strategies is pivotal for enhancing vaccine stability, immunogenicity, and scalability .
3. Expanded Applications of mRNA Vaccine Platforms in Preventing and Controlling Emerging Infectious Diseases
The remarkable success of messenger RNA (mRNA) vaccine platforms, particularly exemplified by their pivotal role in combating the COVID-19 pandemic, has significantly expanded their utility beyond initial expectations, positioning them as a versatile tool for addressing a wide spectrum of infectious diseases and even venturing into therapeutic applications like cancer immunotherapy . This section systematically categorizes and analyzes the expanded applications of mRNA vaccines across different classes of pathogens—viral, bacterial, and parasitic—and explores their burgeoning therapeutic roles. The inherent adaptability and rapid design capabilities of the mRNA platform make it exceptionally well-suited for diverse infectious threats, enabling swift responses to emerging pathogens and the development of targeted interventions .
The first sub-section, "mRNA Vaccine Strategies for Viral Emerging Infectious Diseases," delves into the extensive application of mRNA technology against a broad array of viral pathogens. It highlights the rapid success against SARS-CoV-2 and details ongoing advancements in vaccine development for other high-priority viruses such as influenza, Respiratory Syncytial Virus (RSV), Human Immunodeficiency Virus (HIV), Zika, and Ebola. This sub-section compares the varied antigen selection and immune response goals tailored to the unique characteristics of each virus, from stabilizing prefusion proteins for RSV and SARS-CoV-2 to targeting conserved regions for universal influenza vaccines and inducing broadly neutralizing antibodies for HIV.
The subsequent sub-section, "Prevention of Bacterial and Parasitic Infectious Diseases," addresses the expanding, albeit more challenging, application of mRNA vaccines to bacterial and parasitic pathogens. It outlines the specific immunological complexities posed by these organisms, including their intricate life cycles and diverse immune evasion mechanisms, which necessitate distinct antigen selection and immune response strategies compared to viral targets. This section will also identify critical research gaps and future directions required to overcome these hurdles.
Finally, "Therapeutic Applications Beyond Infectious Diseases" explores the transformative potential of mRNA technology in non-infectious contexts, with a primary focus on cancer immunotherapy and emerging applications in autoimmune diseases and genetic disorders. This sub-section differentiates the mechanisms of action required for therapeutic mRNA from those for prophylactic vaccines, emphasizing the need for nuanced immune modulation to achieve desired therapeutic outcomes. It will also discuss the unique challenges and opportunities associated with these advanced applications, such as personalized neoantigen targeting and precise immune response tuning.
Collectively, this section aims to provide a comprehensive overview of the current landscape of mRNA vaccine applications, underscoring the platform's versatility, rapid adaptability, and significant potential in addressing global health challenges. The comparative analysis of strategies across different disease categories will naturally lead to a discussion of the broader advantages and limitations of the mRNA platform, which will be further elaborated in subsequent sections.
3.1 mRNA Vaccine Strategies for Viral Emerging Infectious Diseases
mRNA vaccine technology has demonstrated remarkable progress against a diverse array of viral pathogens, moving beyond its initial success with SARS-CoV-2 to address other emerging infectious diseases and highly mutable viruses . The rapid adaptability inherent in mRNA platforms is particularly advantageous for countering rapidly mutating viruses and responding swiftly to novel viral threats .
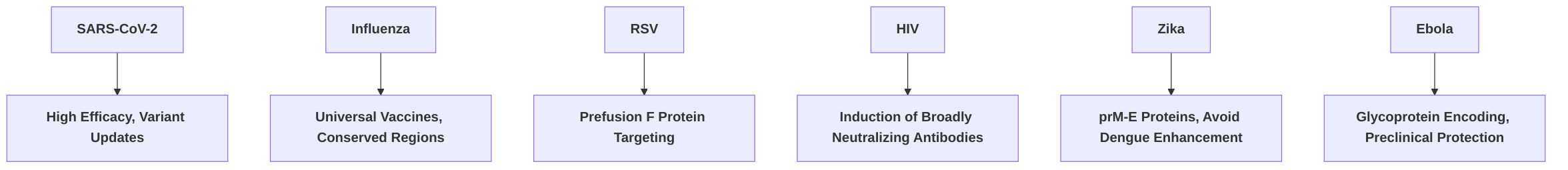
For SARS-CoV-2, two leading mRNA vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), encoding a stabilized prefusion spike protein, achieved high efficacies of 95% and 94.1%, respectively, in preventing symptomatic COVID-19 . These vaccines elicited strong neutralizing antibody responses against various variants, though with reduced effectiveness against strains like B.1.617.1, necessitating booster doses and the development of variant-specific vaccines . The success against SARS-CoV-2 underscores the platform's potential for rapid antigen design and deployment, critical for pandemic preparedness .
Beyond SARS-CoV-2, significant progress has been made in developing mRNA vaccines for other prevalent and emerging viral diseases. For Respiratory Syncytial Virus (RSV), Moderna's mRNA-1345 vaccine, which targets the prefusion F protein, demonstrated an impressive 83.7% efficacy in a late-stage trial for adults aged 60 and over . This strong efficacy highlights the importance of targeting stable, conformationally optimized antigens, such as the prefusion form of the F protein, to elicit robust neutralizing antibody responses.
In contrast, mRNA vaccine development for influenza focuses on strategies to overcome the virus's high mutational rate and antigenic drift. Current research aims to develop universal influenza vaccines by targeting conserved regions, such as the hemagglutinin (HA) stalk, rather than the highly variable HA head region . Multivalent mRNA vaccine candidates for influenza are in Phase I/II trials and have shown strong immunogenicity, with early Phase 1 trials for H10N8 and H7N9 strains inducing high neutralization antibody titers . The observed differences in efficacy between RSV and influenza vaccine candidates could stem from several factors: RSV's F protein offers a more stable and accessible target in its prefusion conformation, which, when stabilized, consistently emits highly potent neutralizing antibodies. Influenza, on the other hand, presents a moving target due to its continuous antigenic drift, necessitating broader immune responses and more complex antigen designs, such as universal vaccines targeting conserved epitopes.
For Human Immunodeficiency Virus (HIV), the challenges are substantial due to the extreme diversity of its envelope protein and the presence of a glycan shield, which complicate the induction of broadly neutralizing antibodies (bNAbs) . Despite these hurdles, mRNA vaccine platforms are being explored to induce bNAbs and robust T cell responses, with clinical trials for mRNA HIV vaccines currently underway, aiming to train the immune system against multiple HIV strains . Recent Phase I trials have indeed shown induction of bNAbs, indicating promising initial steps .
In the context of Zika virus, mRNA vaccines target the prM-E (pre-membrane and envelope) proteins, with design considerations to avoid dengue-enhancing effects through careful antigen presentation and formulation . Phase I candidates have demonstrated acceptable safety and immunogenicity, with preclinical studies showing high neutralizing antibody titers . Similarly, for Ebola virus, mRNA vaccines encoding the glycoprotein have shown efficacy in preclinical studies, leading to effective protection in animal models and increasing titers of Ebola virus glycoprotein serum IgG, suggesting a potentially safer alternative to existing vaccines .
Other viral targets include Rabies virus, for which mRNA vaccines encoding the rabies virus glycoprotein (RABV-G) have progressed to clinical trials, showing promising virus-neutralizing antibody titers and robust antibody and T cell responses . Cytomegalovirus (CMV) is another focus, with mRNA-1647, targeting the pentamer complex and gB antigens, demonstrating high neutralizing antibody titers in Phase 1 and Phase 2 trials . Additionally, early phase trials have demonstrated safety, tolerability, and good immune responses for chikungunya and varicella-zoster virus (VZV) . Combination mRNA vaccines, such as mRNA-1653 targeting the fusion proteins of human metapneumovirus (hMPV) and parainfluenza virus 3 (PIV3), have also shown sustained antibody titers in Phase 1 trials . Epstein-Barr virus (EBV) and Dengue virus are also under investigation, with mRNA vaccine candidates targeting specific glycoproteins in preclinical development for EBV, and ongoing research for Dengue to avoid antibody-dependent enhancement .
The choice of antigen design and optimization strategies plays a crucial role in the effectiveness of mRNA vaccines for specific viruses. Codon optimization, for instance, significantly impacts mRNA translation kinetics and protein expression, thereby influencing immunogenicity. For viruses like RSV and SARS-CoV-2, stabilizing the prefusion conformation of their surface glycoproteins (F protein and spike protein, respectively) through specific mutations or engineering strategies has been critical for inducing robust neutralizing antibody responses. This approach ensures that the vaccine presents the antigen in a conformation most representative of the native viral protein before membrane fusion, maximizing the exposure of neutralizing epitopes. In contrast, for highly diverse viruses like HIV, the strategy shifts towards inducing broadly neutralizing antibodies, which requires more complex antigen engineering, often involving mosaic antigens or sequential immunization regimens to guide germline B cell responses towards conserved epitopes. For influenza, the focus on conserved stalk regions of HA reflects a strategic pivot from traditional head-based vaccines, acknowledging the challenges posed by antigenic drift and aiming for more universal protection. This comparative analysis reveals that while the fundamental mRNA platform remains consistent, the success of vaccine development heavily relies on virus-specific antigen design and optimization to elicit desired immune responses, underscoring the critical interplay between bioinformatics, structural biology, and immunology in mRNA vaccine advancement.
In summary, mRNA vaccine technology has rapidly expanded its applications beyond SARS-CoV-2, demonstrating considerable promise against a broad spectrum of viral pathogens, including influenza, RSV, HIV, Zika, Ebola, CMV, Rabies, and others. The rapid adaptability of this platform, coupled with sophisticated antigen design strategies such as targeting conserved regions, stabilizing prefusion proteins, and leveraging codon optimization, positions mRNA vaccines as a formidable tool in preventing and controlling emerging infectious diseases. While varying efficacies and developmental stages exist across different viral targets, the underlying principles of rapid development, high immunogenicity, and flexibility in design underscore the transformative potential of mRNA vaccines in global health preparedness.
3.2 Prevention of Bacterial and Parasitic Infectious Diseases
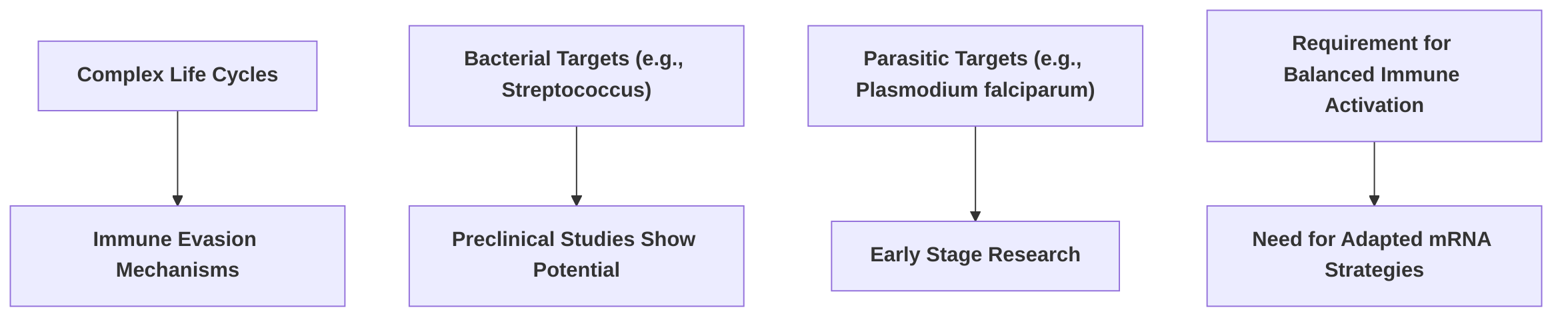
The application of messenger RNA (mRNA) vaccine technology, predominantly successful in viral disease prevention, is expanding to include bacterial and parasitic infections. This expansion presents both considerable potential and unique immunological challenges due to the inherent complexities of these pathogens compared to simpler viral targets . While significant progress has been made in viral vaccine development, the scientific literature indicates a comparatively limited focus on bacterial and parasitic diseases within mRNA vaccine research, highlighting a crucial knowledge deficit .
The structural and genomic characteristics of viruses are generally simpler, facilitating antigen design and streamlining vaccine development . In contrast, bacterial and parasitic pathogens often possess complex life cycles, multiple antigenic variations, and sophisticated immune evasion mechanisms, making the identification of protective antigens and the induction of appropriate immune responses considerably more challenging. For instance, preclinical studies have demonstrated the potential of self-amplifying mRNA (SAM) vaccines against specific bacterial targets such as Streptococcus, specifically targeting Streptolysin-O for Group A Streptococcus (GAS) and pilus 2a backbone protein for Group B Streptococcus (GBS), eliciting immune responses in mice . This represents a promising, albeit early-stage, advancement in bacterial mRNA vaccine development. Furthermore, research has briefly explored an mRNA vaccine targeting Plasmodium falciparum's macrophage migrating inhibitory factor (PMIF) to enhance T cell responses and induce anti-Plasmodium IgG antibodies and memory T cell responses, indicating an initial foray into parasitic disease targets . The potential application for Tuberculosis (TB) has also been briefly mentioned to improve immune responses for enhanced protection . However, these examples are scarce, and comprehensive details on antigen exploration or specific challenges in developing mRNA vaccines for these pathogens remain largely undefined within current literature .
A critical immunological hurdle unique to bacterial and parasitic pathogens lies in the nature of the required protective immune responses. Unlike many viral infections where strong neutralizing antibody responses or cytotoxic T lymphocyte (CTL) activity suffice, bacterial and parasitic infections often necessitate a more complex and balanced immune activation. For example, some bacterial infections may require robust innate immune responses or specific T helper cell subsets (e.g., Th1 or Th17) for effective clearance, while parasitic infections, particularly those with intracellular stages, may demand potent cellular immunity combined with nuanced antibody-mediated mechanisms. The challenge is amplified by the sheer diversity of antigens presented by these organisms and the need to select targets that are both highly immunogenic and broadly protective across different strains or life cycle stages. Current mRNA vaccine strategies, largely optimized for viral antigens, may not inherently elicit these precise immune profiles without substantial adaptation.
The limited understanding of the necessary immune responses and optimal antigen selection for bacterial and parasitic diseases represents a significant knowledge gap. This contrasts sharply with viral targets, where extensive research has delineated key antigenic sites and the immune correlates of protection. For bacterial and parasitic pathogens, identifying vaccine candidates is complicated by factors such as phase variation, antigenic mimicry, and intracellular survival strategies that allow these pathogens to evade host immunity. This necessitates distinct research approaches, moving beyond merely applying existing mRNA vaccine paradigms.
Future research directions must prioritize an in-depth understanding of host-pathogen interactions for bacterial and parasitic diseases to identify crucial immune evasion mechanisms and vulnerable points for vaccine intervention. This includes detailed immunological profiling to determine the precise quality and quantity of immune responses required for protection against diverse bacterial and parasitic threats. Furthermore, exploring novel adjuvant strategies or immunomodulatory approaches specifically designed to bias mRNA-induced immunity towards desired Th1, Th2, Th17, or cytotoxic responses will be essential. This may involve co-delivering mRNAs encoding specific cytokines or immunomodulators alongside the antigen mRNA to shape the immune response.
Addressing these gaps will require significant interdisciplinary collaboration between vaccinology, microbiology, and immunology. Drawing parallels from successful cross-disciplinary approaches in other fields, such as cancer immunotherapy, where a deep understanding of tumor biology, immunology, and drug delivery has led to breakthroughs, could provide a valuable framework. By fostering such collaborations, researchers can bridge the current knowledge divide, develop more effective antigen selection criteria, and design mRNA vaccines capable of eliciting the intricate and robust immune responses required for preventing and controlling bacterial and parasitic infectious diseases, thereby expanding the utility of mRNA vaccine platforms beyond their current viral-centric focus.
3.3 Therapeutic Applications Beyond Infectious Diseases
The success of mRNA vaccine platforms in infectious disease prevention has propelled their exploration into diverse therapeutic applications, most notably cancer immunotherapy . This transition signifies a broader recognition of mRNA technology's versatility, extending its utility beyond prophylactic immunization to active disease treatment. While several reviews primarily focus on the application of mRNA technology for infectious diseases , the limited coverage of therapeutic applications in some literature, such as in , underscores a critical area requiring further in-depth analysis for a comprehensive understanding of the field.
The mechanisms of action required for therapeutic mRNA differ significantly from those of prophylactic vaccines. Prophylactic vaccines aim to induce robust humoral and cellular immune responses against specific pathogens to prevent future infection. In contrast, therapeutic mRNA, particularly in cancer immunotherapy, seeks to stimulate the immune system to recognize and eliminate existing diseased cells, often in an environment characterized by immune evasion and tolerance. For instance, in cancer immunotherapy, mRNA vaccines are designed to encode tumor-associated antigens (TAAs) or neoantigens. These antigens are delivered to antigen-presenting cells (APCs), which subsequently activate cytotoxic CD8+ T cells and helper CD4+ T cells, orchestrating an immune attack against cancer cells .
A key strategy in therapeutic cancer vaccines involves personalized mRNA vaccines, where neoantigens are identified through advanced techniques like next-generation sequencing (NGS), high-throughput screening, and machine learning algorithms to predict optimal patient-specific tumor mutations . This personalized approach contrasts with the broader, population-level target antigens typically used in prophylactic vaccines. Clinical trials are currently evaluating the efficacy of such personalized mRNA vaccines, including BNT111 for melanoma and mRNA-4157 for various solid tumors. Furthermore, mRNA-2752 is being investigated for advanced cancers in combination with immunotherapies like durvalumab . While some early therapeutic efforts, such as protamine-formulated mRNA vaccines (RNActive) for prostate and lung cancers, have shown limited efficacy in survival data, the continuous evolution of vaccine design and adjuvant strategies holds promise . The potential also exists for mRNA vaccines to target viral antigens in virus-associated cancers .
Beyond cancer, mRNA technology is being explored for a range of other therapeutic applications, including autoimmune diseases and genetic disorders . For autoimmune diseases like multiple sclerosis, rheumatoid arthritis, and Type 1 diabetes, the aim is to reprogram immune cells to reduce autoimmune attacks and restore immune tolerance . This requires a nuanced modulation of immune responses, diverging from the strong immunostimulatory effect desired for infectious diseases. In the context of genetic disorders, mRNA therapies deliver instructions for functional proteins, potentially treating conditions like cystic fibrosis or promoting blood vessel growth in heart disease .
The nuances of immune responses elicited by mRNA vaccines are critical for their therapeutic success. The inherent immunostimulatory properties of mRNA, which are beneficial for activating a robust immune response against pathogens, can pose challenges in therapeutic contexts. For instance, in autoimmune diseases, over-activation of the immune system could exacerbate the condition, necessitating strategies to induce immune tolerance rather than overt immunity . Conversely, in cancer immunotherapy, an optimal balance is required to break tumor-induced immune tolerance and generate potent anti-tumor immunity without causing excessive systemic inflammation. This often involves carefully selecting mRNA modifications, delivery systems, and co-formulated adjuvants to steer the immune response toward the desired outcome. The development of combination vaccines addressing multiple pathogens or the integration of personalized RNA mutanome vaccines with existing therapies like PD-1 blockade further exemplifies the innovative approaches to harness mRNA technology for complex therapeutic challenges .
The challenges in these emerging fields include ensuring the precise delivery of mRNA to target cells, overcoming potential off-target effects, and fine-tuning the immune response to avoid undesirable outcomes like immune tolerance in cancer or over-activation in autoimmune diseases . Despite these challenges, the opportunities are substantial. mRNA platforms offer rapid design and manufacturing capabilities, high potency, and the flexibility to encode multiple antigens or therapeutic proteins. This adaptability makes them highly promising for addressing complex diseases that traditional therapeutic modalities have struggled to manage . While comprehensive data on the detailed design, targets, and integration of therapeutic cancer vaccines with other treatments may require supplemental literature, the burgeoning research in this area indicates a transformative potential for mRNA technology . This versatility underscores mRNA's potential to revolutionize not only infectious disease prevention but also a broad spectrum of therapeutic interventions.
4. Advantages and Challenges of mRNA Vaccine Platforms
The rapid ascent of mRNA vaccine platforms marks a significant advancement in vaccinology, fundamentally reshaping approaches to preventing and controlling infectious diseases. This section systematically delineates the inherent strengths of mRNA vaccine technology, such as its unparalleled speed of development and exceptional adaptability, while critically examining the logistical, economic, immunological, and regulatory challenges that currently impede its full global potential . By contrasting mRNA platforms with traditional vaccine methodologies, we highlight their translational advantages. Furthermore, insights derived from the molecular mechanisms and design principles discussed in Chapter 2 will inform a cohesive analysis of the immunological complexities. This comprehensive discussion aims to directly address the review’s objectives by providing a contextual framework for future research and strategic directions.

The "Translational Advantages of mRNA Vaccine Platforms" subsection elaborates on the core benefits that distinguish mRNA vaccines. It highlights the rapid development and manufacturing timelines, evidenced by their swift deployment during the COVID-19 pandemic, attributing this speed to the scalable and adaptable cell-free synthesis process . The discussion also emphasizes the modular design, offering flexibility in antigen selection to combat emerging variants and novel pathogens . Furthermore, the high immunogenicity, inducing robust humoral and cellular immune responses, coupled with an excellent safety profile due to their non-integrating and biodegradable nature, are presented as key advantages .
Following the discussion of advantages, the "Current Challenges and Limitations" subsection delves into significant hurdles for widespread deployment, particularly in resource-limited settings . The primary focus is on the stringent ultra-cold chain storage requirements, which pose substantial logistical challenges, and the high manufacturing costs associated with complex production processes and lipid nanoparticle (LNP) components . This section also highlights the necessity for long-term safety data and the critical impact of public perception and vaccine hesitancy .
The "Immunological Challenges" subsection further refines the understanding of limitations by focusing on the complex immune responses elicited by mRNA vaccines. It addresses the delicate balance between the beneficial immunostimulatory properties of mRNA and the potential for excessive activation, which might diminish effectiveness or induce immune tolerance . This section also considers the need for enhanced mucosal immunity, the durability of protection, and variations in immune responses across diverse demographics, underscoring the necessity for tailored vaccination strategies and continued research into fundamental mechanisms .
Finally, the "Regulatory and Public Perception Challenges" subsection examines the evolving landscape of regulatory oversight and the critical role of public acceptance. It discusses the need for adaptive regulatory frameworks and global harmonization to streamline approval processes for novel mRNA platforms . Concurrently, it addresses vaccine hesitancy and misinformation campaigns as significant barriers to widespread adoption, advocating for interdisciplinary approaches that integrate behavioral economics and communication science to foster public trust and ensure effective vaccine uptake . Collectively, these subsections provide a comprehensive overview of the current standing of mRNA vaccine platforms, highlighting both their transformative capabilities and the critical areas requiring further innovation and strategic intervention for future global health security.
4.1 Translational Advantages of mRNA Vaccine Platforms
The mRNA vaccine platform represents a paradigm shift in vaccinology, primarily owing to its exceptional translational advantages, particularly in the context of rapid response to emerging infectious diseases . A central benefit is the unparalleled speed of development and manufacturing, which was unequivocally demonstrated during the COVID-19 pandemic with the rapid deployment of effective mRNA vaccines . This expedited timeline is largely attributed to the cell-free synthesis process, an enzymatic production method that is both scalable and adaptable, enabling the swift design and synthesis of vaccine candidates within weeks of pathogen identification .
Beyond speed, the modular design of mRNA vaccine platforms confers significant flexibility in antigen selection and design . This inherent adaptability allows for quick modification and redesign in response to the emergence of new pathogen variants or entirely novel infectious agents, a crucial capability in managing rapidly evolving pandemics . For instance, mRNA technology can encode virtually any protein antigen, offering a broad spectrum of possibilities for vaccine development against diverse pathogens . This contrasts sharply with traditional vaccine platforms, which often require extensive cell culture or egg-based production systems that are slower and less adaptable to emergent threats.
Furthermore, mRNA vaccines are characterized by their high immunogenicity, inducing robust humoral and cellular immune responses, alongside the generation of durable immunological memory . This strong immune activation is often attributed to the inherent adjuvant effects of mRNA itself, which can stimulate innate immune pathways . The safety profile of mRNA vaccines is also a significant translational advantage; they do not contain live viral components, eliminating the risk of vaccine-induced infection . Moreover, mRNA is non-integrating, meaning it does not alter the host genome, thereby reducing the risk of genomic alterations and enhancing safety . The biodegradable nature of mRNA ensures that it is naturally degraded and expelled from the body after fulfilling its function, minimizing long-term immune activation or off-target effects . Formulations can also be optimized to achieve desired immunogenicity while minimizing potential adverse effects . These combined attributes underscore the substantial translational benefits of mRNA vaccine technology, positioning it as a pivotal tool for future pandemic preparedness and control of emerging infectious diseases.
4.2 Current Challenges and Limitations
Despite the transformative potential of mRNA vaccine platforms in combating infectious diseases, their widespread deployment, particularly in resource-limited settings, faces significant logistical and economic hurdles . A paramount challenge is the stringent requirement for ultra-cold storage conditions, often necessitating temperatures as low as -70°C for certain formulations . This "cold chain" dependency stems primarily from the inherent instability of mRNA molecules and their lipid nanoparticle (LNP) delivery systems at higher temperatures . Such requirements impede last-mile delivery, especially in regions with inadequate infrastructure and personnel, disproportionately affecting low- and middle-income countries (LMICs) .
Addressing the cold chain challenge necessitates a multifaceted approach focusing on improving thermostability. One promising avenue involves the development of novel LNP formulations designed to maintain mRNA integrity at elevated temperatures, thereby extending shelf life and reducing reliance on ultra-cold storage . Another critical strategy is the application of lyophilization techniques for mRNA-LNP complexes. Lyophilization, or freeze-drying, removes water, stabilizing the vaccine formulation and enabling storage and transport at less demanding temperatures, potentially even room temperature in the future . Furthermore, advancements in temperature-controlled packaging technologies are essential to bridge the gap between current cold chain capabilities and the ideal storage conditions for existing mRNA vaccines, ensuring their viability during transit to remote areas .
Beyond logistical challenges, economic hurdles associated with mRNA vaccine platforms are substantial. The manufacturing process for mRNA vaccines is complex and resource-intensive, requiring high-purity materials and adherence to stringent regulatory standards . This complexity, coupled with the high production costs of LNPs, significantly contributes to the overall expense of mRNA vaccines . The absence of a well-established, standardized manufacturing platform further exacerbates these cost and scalability issues . To mitigate these economic barriers, interdisciplinary approaches are crucial. Process intensification in biomanufacturing can optimize production yields and reduce operational costs. Simultaneously, advanced materials science research can lead to the development of more cost-effective and scalable LNP components, ultimately lowering the overall manufacturing burden and enhancing accessibility, especially in lower-resource regions .
In addition to logistical and economic considerations, the ongoing need for long-term safety and effectiveness data is paramount to building public trust and ensuring sustained impact . While initial reactogenicity, such as fever and headaches, is well-documented , concerns regarding rare adverse events, including potential autoimmune responses linked to spike proteins, necessitate continued rigorous post-market surveillance . Evolving regulatory scrutiny also underscores the demand for comprehensive long-term safety data to facilitate broad acceptance and deployment .
Public perception and vaccine hesitancy constitute another significant hurdle. Misinformation campaigns can lead to widespread distrust and resistance to vaccination, diminishing the public health impact of these novel platforms . Addressing public skepticism requires targeted communication strategies that are transparent, evidence-based, and culturally sensitive, aiming to build confidence and counter misinformation effectively . Furthermore, gaps in knowledge regarding the fundamental mechanisms of action of mRNA vaccines currently limit the development of next-generation vaccines with enhanced potency and safety profiles . Future research should prioritize unraveling these mechanisms to unlock the full potential of mRNA technology and develop more sophisticated, safer, and more effective vaccine candidates. This structured approach to understanding and addressing current challenges will be instrumental in identifying critical areas for future research and development, ultimately paving the way for more equitable and sustainable global deployment of mRNA vaccine platforms.
4.3 Immunological Challenges
The intricate nature of immune responses elicited by mRNA vaccines presents several challenges that necessitate comprehensive understanding and strategic optimization for their expanded applications. While mRNA vaccine platforms are recognized for their robust immunogenicity and ability to induce durable immune responses, including the generation of immunological memory , their interaction with the host immune system is complex and not fully elucidated .
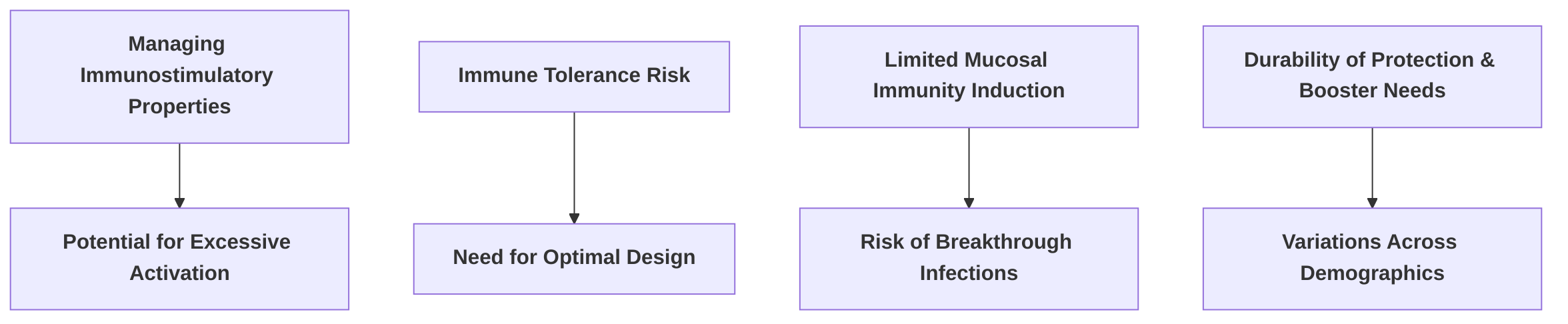
A primary immunological challenge lies in managing the inherent immunostimulatory properties of mRNA. mRNA vaccines activate pattern recognition receptors (PRRs), leading to enhanced Type I Interferon (IFN) production . While this activation is crucial for initiating a robust immune response, excessive or prolonged immune system activation can potentially diminish effectiveness, particularly in therapeutic contexts such as cancer immunotherapy . This delicate balance underscores the need for precise formulation fine-tuning to maximize immunogenicity while mitigating adverse effects .
Furthermore, there is a recognized potential for immune tolerance if mRNA vaccine designs are not optimally configured, which could undermine the desired robust and sustained immune responses, especially for therapeutic applications . The humoral response generated by current mRNA vaccine formulations has not consistently matched that of live attenuated vaccines, indicating a need for further research into vaccine formulations that can achieve superior and more comprehensive immune responses . This concern extends to the induction of mucosal immunity, where mRNA vaccines may not effectively stimulate local immune responses, potentially increasing the risk of breakthrough infections despite systemic protection .
The duration of protection afforded by mRNA vaccines and the long-term effects of repeated dosing remain areas requiring further investigation . The durability of protection is a critical factor, and the necessity for booster immunizations to counteract waning immunity is a notable consideration . This highlights the ongoing need for long-term surveillance studies to inform optimal dosing schedules and ensure sustained protection against evolving pathogens.
Variations in immune responses across different demographics also present significant challenges. Special populations, including immunocompromised individuals, pregnant women, and young children, may exhibit distinct immunological profiles that necessitate tailored vaccination strategies to optimize efficacy and safety . For instance, the elderly or immunocompromised may require specific approaches to elicit an adequately strong and sustained immune response, given their potentially diminished immunological capacity . The efficacy against emerging variants further underscores the dynamic nature of these challenges .
To address these immunological intricacies and optimize immunogenicity, a multidisciplinary approach is essential. This includes integrating findings from immunology to better understand fundamental mechanisms of immune activation and response, and leveraging computational biology to predict immunogenicity based on factors such as antigen design and mRNA modifications. Future research should prioritize the development of advanced computational models capable of predicting individual immune responses. Such models could incorporate genetic factors and prior immune exposure, thereby enabling the tailoring of vaccination strategies for personalized medicine. For instance, understanding the variability in immune responses could be enhanced by developing predictive algorithms for immune efficacy based on individual's HLA types or previous pathogen exposures.
Furthermore, incorporating insights from psychology is crucial for understanding and addressing vaccine hesitancy, which can be influenced by public perceptions of vaccine safety, efficacy, and the severity of potential side effects, including transient inflammatory responses. A holistic understanding that combines immunological rigor with computational predictive power and societal awareness will be pivotal in overcoming current limitations and advancing the application of mRNA vaccine platforms. This comprehensive approach directly supports the review's objective of identifying current challenges and informing strategic directions for future research and development in the field of mRNA vaccines. Continued research is vital to bridge existing knowledge gaps and develop next-generation mRNA vaccines with enhanced potency, safety, and broad applicability .
4.4 Regulatory and Public Perception Challenges
The rapid development and deployment of mRNA vaccines during the COVID-19 pandemic have underscored both their immense potential and the significant challenges associated with their regulatory oversight and public acceptance. The evolving nature of mRNA technology necessitates the continuous adaptation of regulatory frameworks to ensure safety and efficacy, a process that diverges from traditional vaccine approval pathways . While the COVID-19 experience provided an unprecedented volume of data for regulatory processes , the inherent novelty of mRNA platforms, particularly for applications beyond infectious diseases like personalized medicine or gene editing, demands even more flexible and forward-looking guidelines .
A critical aspect of regulatory evolution involves the establishment of new frameworks and global harmonization to streamline the approval process across different jurisdictions . This harmonization is crucial for the efficient global deployment of vaccines, particularly in response to emerging infectious diseases. The lack of extensive long-term safety data for novel mRNA platforms also necessitates sustained post-market surveillance and research to build a comprehensive safety profile . This ongoing scrutiny is essential not only for regulatory assurance but also for fostering public confidence.
Beyond regulatory complexities, public perception issues, including vaccine hesitancy and the pervasive spread of misinformation, represent substantial barriers to the widespread adoption and effectiveness of mRNA vaccines . These challenges significantly impact vaccine uptake and, consequently, public health outcomes . Public skepticism often arises from a lack of understanding regarding the underlying science, compounded by targeted misinformation campaigns that exploit uncertainties and sow distrust . While regulatory guidelines are meticulously addressed in the development and approval process, the equally critical aspect of public perception has historically received less dedicated research, highlighting a crucial gap that needs to be filled for future vaccine strategies .
To effectively mitigate vaccine hesitancy, strategies must extend beyond conventional public health communication. Integrating insights from behavioral economics and communication science can provide a more nuanced understanding of the factors influencing vaccine acceptance. Behavioral economics, for instance, can illuminate cognitive biases and heuristics that shape individuals' risk perceptions and decision-making regarding vaccination. For example, the availability heuristic might lead individuals to overestimate rare adverse events if such instances are widely publicized, even if statistically insignificant. Similarly, framing effects can influence how vaccine efficacy and safety data are perceived; presenting benefits in terms of lives saved rather than disease reduction rates might elicit a stronger positive response.
Communication science offers frameworks for developing more effective and empathetic public health messaging. This involves moving away from a purely didactic approach, which often focuses solely on delivering scientific facts, towards a more dialogic and community-centered engagement. Strategies should consider tailoring messages to specific cultural contexts and addressing the unique concerns of different demographic groups. For instance, engaging trusted community leaders and healthcare professionals in vaccine advocacy can significantly enhance message credibility and foster trust at a local level. The World Health Organization (WHO), with its established reputation, is uniquely positioned to counteract misinformation by leveraging its authority and reach to disseminate accurate, evidence-based information .
Furthermore, lessons learned from recent pandemic experiences underscore the importance of transparency in data sharing, especially concerning long-term safety and surveillance data . Open communication about the uncertainties inherent in novel technologies, coupled with clear explanations of risk-benefit profiles, can build a foundation of trust. Proactive engagement with public concerns, rather than reactive responses to misinformation, is essential. This includes preemptively educating communities about vaccine safety and efficacy before widespread deployment, thereby preparing the public for future vaccination campaigns .
In conclusion, the successful expansion of mRNA vaccine applications hinges on robust and adaptable regulatory frameworks that can accommodate technological advancements and global collaborative efforts for approval and deployment. Simultaneously, addressing public perception challenges requires a sophisticated, interdisciplinary approach that moves beyond traditional public health communication. By integrating insights from behavioral economics and communication science, researchers and policymakers can develop more effective strategies to enhance public trust, mitigate vaccine hesitancy, and ensure the optimal uptake of these critical interventions in preventing and controlling emerging infectious diseases. Future research should prioritize understanding the psycho-social determinants of vaccine acceptance and developing tailored communication interventions that resonate with diverse populations.
5. Future Directions and Pandemic Preparedness
This section delves into the promising future advancements in messenger RNA (mRNA) vaccine technology, building upon the foundations of current platforms and addressing their limitations. It systematically explores innovations poised to revolutionize vaccine efficacy, stability, and administration. The discussion will cover emerging technologies and innovations, including advancements in thermostable formulations and novel delivery systems, as well as the development of self-amplifying and circular RNA constructs . Furthermore, the section will examine the strategic integration of mRNA vaccines with other therapeutic modalities and the potential for mRNA-encoded adjuvants to enhance immune responses . Finally, it will analyze the pivotal role of mRNA technology in bolstering global pandemic preparedness, emphasizing rapid response capabilities, equitable access initiatives, and collaborative frameworks for future health security . This comprehensive overview will highlight how these advancements could overcome current limitations and expand the platform's utility for future pandemic preparedness and equitable global health initiatives, ultimately providing a conclusive summary of the field's trajectory.
5.1 Emerging Technologies and Innovations
Next-generation mRNA technologies hold significant promise for revolutionizing vaccine efficacy, stability, and administration, presenting a clear trajectory for future advancements beyond current platforms. Current research is actively exploring various innovative mRNA vaccine platforms to address existing limitations and expand their applicability. One prominent area of development is the pursuit of thermostable mRNA formulations, aiming to mitigate or eliminate the reliance on stringent cold chain requirements for storage and distribution . This is crucial for global vaccine accessibility, particularly in resource-limited settings. Lyophilized (freeze-dried) mRNA vaccines are specifically being investigated as a means to achieve room-temperature shelf stability, thereby improving logistical feasibility and patient compliance .
Beyond formulation, significant efforts are directed towards optimizing delivery methods to enhance patient compliance and expand administration options. Alternative delivery routes such as oral, intranasal, and microneedle patches are under investigation to provide needle-free vaccination, which could greatly improve patient acceptance and ease of administration . Advancements in lipid nanoparticle (LNP) formulations are central to these innovations, focusing on developing next-generation LNPs with enhanced targeting capabilities, improved cellular uptake, and reduced toxicity, while also facilitating endosomal release of mRNA . The integration of novel materials for tissue-specific targeting within LNP formulations is also enhancing overall safety and efficacy .
Emerging mRNA platform technologies are also being explored for their potential to enhance immunogenicity and reduce dosage requirements. Self-amplifying RNA (saRNA) is a key innovation in this domain, distinguished by its ability to replicate within host cells, leading to sustained protein expression and requiring significantly lower mRNA doses for therapeutic effects compared to traditional mRNA vaccines . This feature of saRNA offers a substantial benefit in terms of manufacturing cost and scalability. Furthermore, circular RNA (circRNA) is under active investigation as a more stable alternative to linear mRNA, promising enhanced stability and potentially reduced immune interference due to its covalently closed loop structure, which renders it resistant to exonuclease degradation . While saRNA can replicate and circRNA offers enhanced stability, both represent significant advancements over current linear mRNA platforms by potentially improving immunogen expression and longevity.
In addition to these platform advancements, future research directions include the development of mRNA-encoded adjuvants. Incorporating adjuvant properties directly into the mRNA sequence could reduce the need for co-administered molecules, simplifying vaccine formulations and potentially enhancing the immune response in a more integrated manner. This approach aligns with the broader goal of optimizing mRNA design through sequence optimization, UTR modifications, and the incorporation of modified nucleosides to enhance stability, translation efficiency, and reduce immunogenicity .
The application scope of mRNA technology is also expanding rapidly beyond conventional infectious disease prevention. Personalized mRNA vaccines tailored to individual genomic information, particularly for cancer treatment targeting specific tumor mutations or neoantigens, represent a cutting-edge development that could be adapted for highly specific infectious disease scenarios . Research is also expanding mRNA applications to non-infectious diseases such as autoimmune disorders and genetic conditions, aiming to induce immune tolerance or deliver therapeutic proteins, showcasing the versatility of the mRNA platform . The development of combination vaccines that address multiple pathogens in a single formulation, such as universal flu vaccines or combined COVID-19 and influenza vaccines, signifies another crucial area of innovation for broader public health impact .
In summary, the next generation of mRNA technologies promises significant leaps in vaccine development. The shift towards thermostable formulations, diversified delivery methods including microneedle patches, the exploration of self-amplifying and circular RNA platforms, and the potential for mRNA-encoded adjuvants collectively aim to improve vaccine efficacy, stability, and ease of administration. These innovations address key challenges of current mRNA platforms, particularly concerning cold chain requirements and patient compliance, while also paving the way for lower dosing, sustained expression, and broader applicability. These advancements are crucial for developing more potent, accessible, and versatile vaccines, thereby outlining robust future directions for the field of infectious disease prevention and control .
5.2 Combination Strategies and Adjuvants
The inherent flexibility and immunogenic properties of messenger RNA (mRNA) vaccine platforms present significant opportunities for synergistic integration with other therapeutic strategies, particularly in the fields of infectious disease prevention and cancer immunotherapy. While some studies do not extensively detail combination strategies or the role of adjuvants in mRNA vaccines , a growing body of research highlights their potential. The literature indicates a clear trend towards exploring these combinations to enhance immune responses and therapeutic outcomes .
One promising area of integration involves combining mRNA vaccines with other immunomodulators. For instance, in cancer immunotherapy, mRNA vaccines have been explored in conjunction with immune checkpoint inhibitors (ICIs) to improve therapeutic efficacy . This approach leverages the ability of mRNA vaccines to prime and activate anti-tumor immune responses, while ICIs, such as anti-PD-1 or anti-PD-L1 antibodies, overcome immune suppression in the tumor microenvironment. Specific innovative strategies include the delivery of mRNA encoding the tumor suppressor p53 via nanoparticles, combined with anti-PD-1 antibodies, to effectively remodel the tumor microenvironment . Furthermore, mRNA vaccines encoding immune regulatory factors are being investigated in combination with antibodies like durvalumab, underscoring a multifaceted approach to augmenting therapeutic responses . This integration capitalizes on mRNA's capacity to deliver instructions for antigen production, thereby expanding the repertoire of immune targets and enhancing T-cell activation.
Beyond immunomodulators, the integration of mRNA vaccines with existing vaccine technologies is also under consideration to achieve more robust and durable protection . While the specifics of such combinations are not universally detailed across all reviewed papers, the general consensus points towards leveraging the strengths of different vaccine modalities. For instance, a combined COVID-19 and influenza vaccine formulated as a single mRNA product exemplifies a direct combination strategy to address multiple pathogens simultaneously . This "multi-antigen" approach simplifies immunization schedules and could significantly improve vaccine coverage and public health outcomes.
Regarding adjuvants, the discussion within the literature is nuanced. The inherent immunostimulatory properties of mRNA itself are frequently highlighted, often serving as a natural adjuvant due to its recognition by pattern recognition receptors, particularly Toll-like receptors (TLRs) . This intrinsic immunostimulation can induce a robust innate immune response, which is crucial for subsequent adaptive immunity. Despite this, the explicit use of external adjuvants to further boost immunogenicity of mRNA vaccines is still considered and explored . While specific adjuvants are not extensively detailed in the provided digests, the general principle is that carefully selected adjuvants could optimize the magnitude, quality, and breadth of the immune response, especially for antigens that are less immunogenic on their own or in populations with compromised immune systems. The rationale for adding adjuvants typically revolves around enhancing antigen presentation, prolonging antigen exposure, or directing the immune response towards a more desirable T helper 1 (Th1) or Th2 profile.
The development of broad-spectrum or universal vaccines represents a significant frontier for mRNA platforms. This feasibility stems from mRNA technology's rapid design and manufacturing capabilities, coupled with its capacity to encode multiple antigens or conserved epitopes. The concept of "multi-antigen" and "pan-vaccine" approaches is actively being pursued to provide broader protection against diverse strains or even entire families of pathogens . For instance, the development of universal influenza vaccines and pan-coronavirus vaccines is a key objective, achieved by targeting highly conserved antigens or shared epitopes across different viral variants . This strategy aims to overcome the limitations of conventional strain-specific vaccines, which often require frequent updates due to rapid viral evolution. The ability to rapidly synthesize mRNA encoding various conserved regions or mosaic antigens makes mRNA platforms uniquely suited for such broad-spectrum applications, potentially offering long-lasting and more comprehensive protection against unpredictable outbreaks and future pandemics. This includes the potential for personalized mRNA vaccines for cancer immunotherapy, tailoring treatments to individual patient tumor profiles .
In summary, while the intrinsic immunostimulatory properties of mRNA mitigate the absolute necessity of external adjuvants in some contexts, the deliberate integration of mRNA vaccines with other immunomodulators, existing vaccine platforms, and potentially specific adjuvants is being explored to maximize immune responses and broaden protective capabilities. The inherent versatility of mRNA technology is also paving the way for the development of multi-antigen and pan-vaccine strategies, marking a significant step towards more resilient and comprehensive public health interventions against emerging infectious diseases.
5.3 Role in Global Pandemic Preparedness
The transformative potential of mRNA vaccines in future pandemic responses is underscored by their inherent capacity for rapid antigen switching and large-scale production, a capability profoundly demonstrated during the COVID-19 pandemic . This characteristic positions mRNA technology as a fast and adaptable solution for tackling novel viral threats and responding swiftly to emerging infectious diseases . The modular nature of mRNA platforms facilitates quick adaptation to new variants, offering the prospect of broader protection against evolving pathogens . Consequently, future preparedness strategies include the development of mRNA vaccine libraries for known high-threat pathogens, enabling faster deployment during outbreaks, and the exploration of "Disease X" investments to preemptively address unknown future pandemics .
Despite these technological advantages, ensuring global equitable access and overcoming geopolitical challenges in vaccine distribution remain critical considerations for effective pandemic preparedness . Challenges such as stringent cold chain requirements, limitations in manufacturing and distribution capacity, public hesitancy, and economic barriers impede the equitable deployment of mRNA vaccines, particularly in underserved regions . To address these multifaceted issues, several actionable strategies are imperative. First, investing in and expanding manufacturing infrastructure in underserved regions is crucial, particularly through initiatives such as the WHO mRNA technology transfer program, which aims to build local capacity and foster rapid response mechanisms for future pandemic threats .
Second, the establishment of global "biofoundries" for on-demand mRNA vaccine production, coupled with the stockpiling of prepositioned mRNA vaccine libraries targeting known high-threat pathogens, offers a robust framework for quick deployment during outbreaks . Third, fostering robust international collaboration is essential to harmonize regulatory frameworks and streamline global distribution mechanisms, ensuring that vaccines reach populations in need efficiently and effectively . This collaboration extends to integrating mRNA vaccine technology into existing public health frameworks to enhance global health security and preparedness, as highlighted by the importance of cooperation among researchers, governments, and communities, especially in addressing regional pathogens like those prevalent in Africa . Furthermore, improving delivery systems and enhancing thermal stability of mRNA vaccines are critical future efforts to strengthen global health security and equitable access, particularly in challenging environments .
Beyond viral threats, future research directions must encompass understanding and overcoming the unique immunological barriers presented by bacterial and parasitic infections. This necessitates interdisciplinary collaboration between vaccinology, microbiology, and immunology. Drawing parallels from successful strategies in other fields, such as those discussed in the context of cancer immunotherapy, or leveraging the foundational principles of mRNA technology, could provide novel insights . For instance, the flexible nature of mRNA platforms might allow for the presentation of multiple bacterial or parasitic antigens, potentially overcoming the complex immune evasion mechanisms characteristic of these pathogens. This approach could involve combining mRNA-based antigen delivery with adjuvants or immunomodulators to elicit potent and protective immune responses that have historically been challenging to achieve with traditional vaccine platforms.
Moreover, the integration of advanced data analytics from computer science is pivotal for real-time surveillance and predictive modeling of emerging threats, which can optimize mRNA vaccine deployment strategies. Such analytical capabilities can help identify potential outbreaks early, predict pathogen evolution, and guide the rapid design and manufacturing of targeted mRNA vaccines. This direct linkage between scientific innovation, strategic infrastructure, equitable access policies, and advanced computational tools forms a comprehensive framework for bolstering global pandemic preparedness. The successful integration of these elements will not only mitigate the impact of future pandemics but also foster a more resilient and equitable global health landscape.
6. Conclusion
This review systematically synthesizes the transformative impact of messenger RNA (mRNA) vaccine platforms on global health, delineating their current achievements and future potential.
The revolutionary attributes of mRNA technology, including its unparalleled speed of development, remarkable efficacy, and inherent adaptability, have fundamentally reshaped the landscape of infectious disease prevention and control, most notably evidenced by its pivotal role during the COVID-19 pandemic . Significant advancements in delivery systems, particularly lipid nanoparticles (LNPs), have been instrumental in ensuring robust immunogenicity and a favorable safety profile . Beyond SARS-CoV-2, the platform has demonstrated remarkable promise against a diverse array of pathogens, encompassing emerging viral threats like Zika, Ebola, influenza, and RSV, and extending its utility to bacterial pathogens and complex diseases such as HIV .
Crucially, the scope of mRNA technology transcends infectious diseases, with significant applications emerging in cancer immunotherapy, where it facilitates targeted immune responses against tumor cells by encoding tumor-specific antigens and neoantigens . This diversification into personalized medicine, including potential applications in autoimmune disorders and genetic conditions, underscores its long-term transformative potential . The development of multi-pathogen vaccines, capable of encoding multiple antigens, represents a key future prospect for streamlined immunization .
Despite these profound advantages, several challenges persist that require dedicated research and development. Foremost among these are the stringent cold chain requirements for storage and distribution, which pose substantial logistical and cost hurdles, particularly in low-resource settings . Future research must prioritize the development of more thermostable formulations and novel delivery mechanisms . Enhancing the durability and breadth of immune responses, along with a deeper understanding of mRNA mechanisms of action, are critical for optimizing vaccine potency and safety . Long-term safety and efficacy data gaps necessitate continued post-market surveillance . Scaling up global manufacturing capacity and reducing production costs are essential for ensuring equitable access, particularly for low-income countries, necessitating strategic initiatives like the WHO mRNA technology transfer hub . Finally, addressing public perception and vaccine hesitancy through transparent communication is vital for maintaining trust and widespread acceptance .
In conclusion, mRNA vaccine technology represents a cornerstone of future immunization and disease control strategies, poised to revolutionize medicine beyond infectious diseases. The ongoing research and strategic collaborations addressing the identified challenges will be crucial in fully realizing its vast potential and ensuring global health security .
6.1 Summary of Key Findings
The review underscores the profound and transformative impact of messenger RNA (mRNA) vaccine technology on global health, fundamentally altering the landscape of infectious disease prevention and control. A central argument consistently highlighted across various studies is the revolutionary nature of mRNA vaccines, primarily attributed to their unprecedented speed of development, remarkable efficacy, and inherent adaptability . This platform's ability to rapidly synthesize vaccine candidates upon sequence identification has proven pivotal, particularly during the acute phase of the COVID-19 pandemic, where it demonstrated rapid development and deployment capabilities unprecedented in vaccinology .
The success of mRNA technology is inextricably linked to significant advancements in delivery systems, particularly lipid nanoparticles (LNPs), which efficiently encapsulate and deliver mRNA into target cells, ensuring high immunogenicity and robust immune responses . This robust immunogenicity, coupled with the non-integrating nature of mRNA into the host genome, confers a significant safety advantage over traditional vaccine platforms . The inherent flexibility of the mRNA platform allows for rapid antigen design and modification, enabling broad applicability against a diverse range of pathogens, including emerging and re-emerging infectious diseases .
Beyond SARS-CoV-2, mRNA vaccine platforms have shown remarkable promise and successful application against various viral emerging infectious diseases (EIDs), including Zika, Ebola, influenza, and Respiratory Syncytial Virus (RSV) . Furthermore, the technology extends its utility beyond viral threats to encompass bacterial pathogens like Streptococcus, and demonstrates considerable potential in tackling complex diseases such as HIV . This broad spectrum of efficacy highlights the platform's versatility and its critical role in enhancing global pandemic preparedness and response capabilities .
A crucial finding is the expanded application of mRNA technology beyond infectious diseases. Several studies emphasize its significant promise in cancer immunotherapy, where it stimulates targeted immune responses against tumor cells by encoding tumor-specific antigens and neoantigens . This diversification into personalized medicine and other non-infectious disease areas, including autoimmune disorders and genetic conditions, represents a substantial future direction and underscores the technology's long-term transformative potential . The concept of multi-pathogen vaccines also emerges as a key future prospect, leveraging the platform's ability to encode multiple antigens simultaneously .
Despite these profound advantages and successes, significant challenges remain, necessitating concerted efforts for their mitigation. A recurring challenge is the requirement for stringent cold chain logistics, which poses substantial hurdles for global deployment, particularly in low- and middle-income countries (LMICs) and regions like Africa, where infrastructure limitations are pronounced . Issues related to mRNA instability and the need for improved delivery systems also persist .
Furthermore, ensuring manufacturing scalability and cost-effectiveness remains critical for widespread and equitable access, especially for global health initiatives. The cost implications and the need for scalable production facilities are frequently cited as barriers to broader implementation . Public perception and acceptance also constitute a significant challenge, requiring robust communication strategies to address misinformation and build trust .
In conclusion, the review consistently highlights mRNA vaccine technology as a pivotal innovation, capable of transforming global health through its unparalleled speed, efficacy, and adaptability. Its proven success against COVID-19 has not only validated its potential but also accelerated research and development across a myriad of infectious diseases and into novel therapeutic areas like cancer. While challenges in logistics, cost, and public acceptance persist, ongoing research and strategic collaborations are poised to overcome these hurdles, further cementing mRNA's role as a cornerstone of future immunization and disease control strategies. The future trajectory for mRNA platforms includes significant expansion into personalized medicine, multi-pathogen vaccines, and rapid response capabilities for unforeseen emerging pathogens, reinforcing its transformative impact on global health.
6.2 Remaining Challenges and Future Outlook
Despite the transformative potential demonstrated by mRNA vaccine platforms, particularly during the COVID-19 pandemic, several critical research gaps and unresolved questions persist, necessitating further investigation to fully realize their capabilities . A primary challenge lies in improving the intrinsic stability of mRNA molecules and optimizing their delivery systems, which are often reliant on lipid nanoparticles (LNPs) . This reliance on LNPs currently mandates stringent cold chain requirements for storage and distribution, presenting significant logistical hurdles and increasing costs, particularly for last-mile delivery in low-resource settings . Future research efforts must prioritize the development of more thermostable formulations, such as lyophilized or room-temperature stable mRNA vaccines, and explore alternative delivery mechanisms to circumvent these cold chain dependencies .
Another critical area of investigation involves enhancing the durability and breadth of immune responses elicited by mRNA vaccines . A deeper understanding of the mechanisms of action is required to increase vaccine potency and safety, facilitating rational antigen design, innovative delivery strategies, and optimized vaccination regimens . While mRNA vaccines have shown remarkable efficacy, concerns regarding potential immunological side effects and the fine-tuning of their immunogenicity remain . Research into novel mRNA technologies, such as self-amplifying RNA (saRNA), could offer pathways to improved efficacy with lower doses, potentially mitigating some of these concerns . Furthermore, the long-term safety and efficacy data gaps need to be addressed through continued surveillance and post-market studies .
Scaling up manufacturing capacity globally and reducing production costs are significant challenges that must be overcome to ensure equitable access to mRNA vaccines, especially for low-income countries . Strategic initiatives, such as the WHO mRNA technology transfer hub, are crucial steps towards broadening access and building decentralized manufacturing capabilities to prepare for future health threats . Simultaneously, public perception and vaccine hesitancy present substantial obstacles that require sustained educational efforts and transparent communication to build and maintain public trust .
Looking forward, the trajectory of mRNA vaccine technology is profoundly promising, positioning it to revolutionize vaccinology and medicine . The expansion of mRNA applications beyond viral infectious diseases to include bacterial and parasitic pathogens is a key future direction, broadening the scope of its impact on global health . The adaptability and rapid development capabilities of mRNA platforms make them ideal candidates for integration into robust pandemic preparedness strategies, enhancing global health security .
Beyond infectious diseases, mRNA technology is poised to become a cornerstone of precision medicine, offering innovative solutions to a wider array of medical challenges, including cancer immunotherapy, autoimmune disorders, and genetic diseases . The ability to leverage mRNA technology for personalized medicine, tailoring vaccines or therapies to individual patient needs, represents a significant paradigm shift in healthcare . Furthermore, the development of combination vaccines, delivering antigens for multiple pathogens simultaneously, could streamline immunization schedules and improve global vaccine coverage . The ongoing research into novel mRNA platforms, optimized delivery systems, and sophisticated bioinformatics tools will continue to refine mRNA design and accelerate vaccine development, ultimately contributing to improved public health resilience and disease combat on a global scale .
References
mRNA vaccine platforms: linking infectious disease prevention and cancer immunotherapy https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1547025/full
mRNA Vaccines & Pandemic Preparedness: Future Applications Beyond COVID-19 https://uppcsmagazine.com/mrna-vaccines-pandemic-preparedness-future-applications-beyond-covid-19/
Potential benefits and limitations of mRNA technology for vaccine research and development for infectious diseases and virus-induced cancers - World Health Organization (WHO) https://iris.who.int/bitstream/handle/10665/375031/9789240084551-eng.pdf
mRNA vaccine platforms: linking infectious disease prevention and cancer immunotherapy - Frontiers https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1547025/full
Application of mRNA Vaccine Platforms for Emerging Infectious Diseases: Progress and Challenges https://www.ijcmas.com/14-6-2025/Densingh%20Johnrose,%20et%20al.pdf
The role of mRNA vaccines in infectious diseases: a new era of immunization https://www.springermedizin.de/the-role-of-mrna-vaccines-in-infectious-diseases-a-new-era-of-im/50981958
The Development of mRNA Vaccines for Infectious Diseases: Recent Updates - PMC https://pmc.ncbi.nlm.nih.gov/articles/PMC8668227/
mRNA vaccines for infectious diseases - advances, challenges and opportunities - PubMed https://pubmed.ncbi.nlm.nih.gov/39367276/
